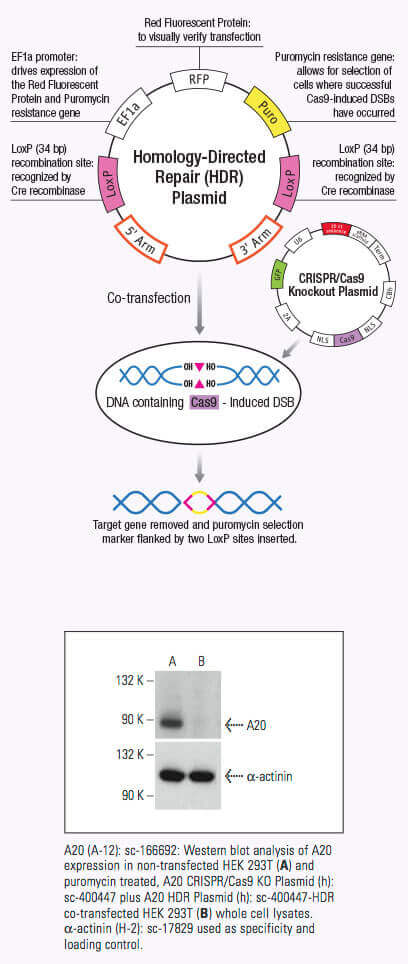
Systèmes CRISPR
Santa Cruz Biotechnology propose désormais des plasmides CRISPR/Cas9 Knockout (KO) spécifiques à une cible, des plasmides CRISPR Double Nickase, des plasmides d'activation CRISPR/ dCas9 et des systèmes d'activation CRISPR Lenti pour plus de 18 910 gènes codant pour des protéines humaines et 18 340 gènes codant pour des protéines de souris.
Quick Links
Historique CRISPR/Cas9
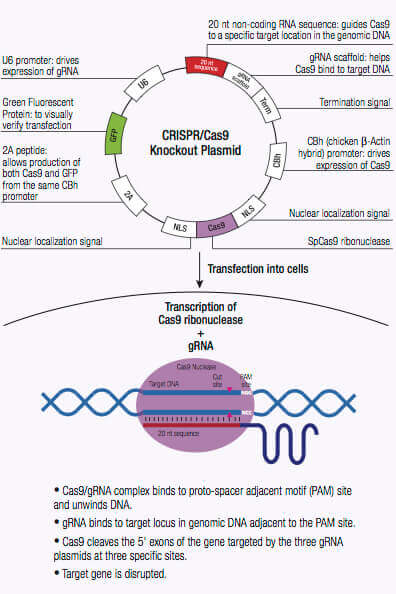
Le système CRISPR/Cas est un mécanisme de défense immunitaire adaptatif utilisé par les bactéries et les lignées Archea pour la dégradation du matériel génétique qui leur est étranger. Dans ces organismes, le matériel génétique étranger provenant d'un bactériophage est acquis et intégré dans le locus CRISPR (1,2). Ce nouveau matériau, aussi connu comme un élément d'espacement, crée un fragment spécifique de la séquence utilisée pour la résistance contre une future infection bactériophage. Ces fragments spécifiques d'une séquence sont convertis en ARNs courts CRISPR (crARNs) et fonctionnent comme guide pour diriger le clivage de l'ADN complémentaire invasif par l'activité nucléase de la protéine associée-CRISPR (Cas) également codée par le locus CRISPR (1,2). La protéine nucléase Cas9 du système de CRISPR de type II possède un domaine de liaison à l'ARN, un lobe de reconnaissance d'hélice alpha (REC), un lobe de nucléase qui comprend le RuvC et HNH pour le clivage de l'ADN, et un site d'interraction du motif adjacent de protospacer (PAM) (1,2). Le crARN forme un complexe avec la nucléase Cas9 en se liant au lobe de REC, et forme plusieurs ponts salins avec le backbone du crARN (1,2,3).
Une fois que le crARN se lie à la Cas9, la conformation de la nucléase Cas9 change et crée un canal permettant la liaison de l'ADN (1,2,3). Le complexe Cas9/crARN analyse l'ADN pour trouver un site PAM (5'-NGG) (4,5,6). La reconnaissance d'un site PAM conduit au déroulement de l'ADN, et permet au crARN de vérifier la complémentarité de l'ADN adjacent au site PAM. Lorsque Cas9 se lie à un site PAM adjacent à une séquence d'ADN complémentaire du crARN, le lobe REC crée un hétéroduplex ARN-ADN avec l'ADN cible (3,4,7). La reconnaissance du site PAM est impliquée dans l'activation des domaines HNH et RuvC nucléolytiques qui entraine une coupure du double brin (DSB) dans l'ADN cible, conduisant à la dégradation de l'ADN (1,2,5,8). Si le crARN n'est pas complémentaire, alors Cas9 libère et recherche un autre site PAM (7). Les ruptures ciblées des brins du génome dans l'ADN peuvent être réparées via la voie de réparation des extrémités de jonction non homologues (NHEJ), qui entraine des insertions ou des suppressions créant des erreurs, ou par la voie de réparation homologue dirigée (HDR), qui peut être utilisée pour recombiner les marqueurs sélectionnés à des sites spécifiques dans le génome (2,9,10). Ce mécanisme CRISPR/Cas9 peut être réutilisé pour l'ingénierie génomique de divers systèmes, y compris les cellules mammaliennes.
La modification du génome via l'introduction de DSBs peut être réalisée avec des méganucléases, des nucléases à doigts de zinc (ZF), ou des effecteurs transactivateurs (TALEs), qui reconnaissent des séquences d'ADN, cependant, chacune de ces techniques a ses limites. Lors de l'utilisation de méganucléases il est difficile de montrer clairement la reconnaissance spécifique de site entre la nucléase et l'ADN (2). Les autres options, ZFs et TALEs, se sont avérées difficiles à concevoir et à reconnaître plus de 3 nt de l'ADN (2). Les ARN guides simples (sgARNs) qui agissent comme les crARNs sont facilement conçus et peuvent être exprimés avec la nucléase Cas9 dans le même vecteur pour cibler des sites spécifiques de l'ADN pour la modification du génome. Le système CRISPR/Cas9 dispose également d'une plus grande sensibilité et est plus efficace lorsqu'il est utilisé pour le screening que les petits shARN (small hairpin ARNs).
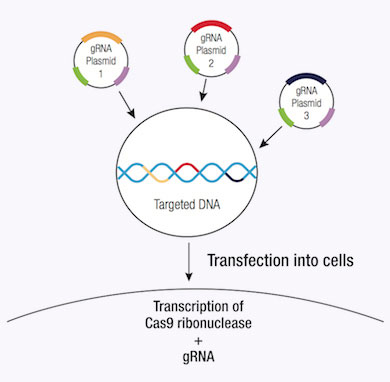
L'avantage principal de l'utilisation du système CRISPR/cas9 pour induire le DSB dans l'ADN génomique est son haut niveau d'efficacité. Cependant, cette efficacité peut être obscurcie par un certain nombre d'effets hors-cible, réduisant ainsi la spécificité de ce CRISPR/cas9. La spécificité peut être améliorée en utilisant un système CRISPR double nickase, dans lequel une paire de plasmides, chacun codant pour un cas9 (D10A) mutant nickase (Cas9n), sont dirigés vers un site spécifique d'une région distinct de l'ADN génomique grâce à un ARN guide spécifique de la cible (12). Chaque complexe Cas9n/sgARN crée une seule entaille dans le brin d'ADN qui est complémentaire de l'ARN guide (12). Chaque paire d'ARN guide est décalée d'environ 20 pb et reconnaît les séquences cibles situées sur les brins opposés de l'ADN cible. La double entaille créée par la paire de complexes Cas9n/ARNsg imite un DSB (12). Ainsi, l'utilisation de paires d'ARN guide permet d'accroitre la spécificité de l'édition du gènecible, tout en maintenant un haut niveau d'efficacité (12).
En plus de la modification du génome, le système de CRISPR a été conçu pour permettre l'activation significative de l'expression du gène endogène (13). Plusieurs composants du système de CRISPR ont été modifiés pour générer le complexe médiateur d'activation synergique (SAM) qui aboutit à un système d'activation de la transcription hautement efficace et spécifique (13). L'un des composants du complexe SAM modifié est la nucléase Cas9. Dans le système SAM, les domaines catalytiques de Cas9 sont désactivés et le dCas9 résultant est fusionné à un domaine d'activation de transcription (VP64). Dirigé par un ARN guide (sgRNA) cible, le complexe dCas9-VP64-sgRNA cible la région de 200pb du Site Transcriptionel de Départ (TSS) des gènes cellulaires pour réguler positivement l'expression de ces gènes (13). Pour améliorer la transcription, le sgARN a été modifié en y ajoutant un aptamère hairpin (épingle à cheveux) à la tétraboucle et à la tige de la boucle 2 (13). Ce aptamère sur le sgARN se lie sélectivement aux protéines d'enveloppe du bactériophage MS2 dimérisés (13). Fusioner les protéines MS2 aux domaines de transactivation p65 et HSF1 permet à la protéine de fusion résultante MS2-P65-HSF1 d'améliorer le recrutement de facteurs de transcription, améliorant ainsi la puissance de l'activation de gènes médié par dCas9 (13).
Quick Links
CRISPR/Cas9-Cassure Dirigée du Double Brin (DSB)