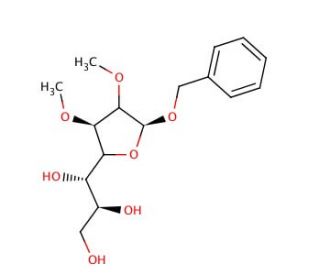

Benzyl 2,3-O-Isopropylidene-L-glycero-α-D-mannoheptofuranoside
QUICK LINKS
Benzyl 2,3-O-Isopropylidene-L-glycero-α-D-mannoheptofuranoside is a key chemical compound extensively utilized in carbohydrate chemistry research, particularly in the synthesis of complex carbohydrates and glycoconjugates. Its mechanism of action primarily involves its role as a protecting group in carbohydrate chemistry. The introduction of the benzyl 2,3-O-isopropylidene moiety serves to protect the hydroxyl groups on the carbohydrate molecule, preventing undesired reactions at these positions during subsequent chemical manipulations. This protection strategy enables chemists to selectively modify other functional groups on the carbohydrate scaffold, facilitating the synthesis of diverse carbohydrate derivatives with precise control over regio- and stereochemistry. Furthermore, Benzyl 2,3-O-Isopropylidene-L-glycero-α-D-mannoheptofuranoside has been instrumental in the development of novel glycosylation methodologies, including glycosylation reactions mediated by metal catalysts or enzymes. Its utility extends to the synthesis of glycoconjugates for various research applications, such as the construction of glycopeptides, glycolipids, and glycoproteins for studying carbohydrate-protein interactions, cell surface recognition processes, and the role of glycans in biological systems. Additionally, its versatility and reliability make it a valuable tool in glycobiology research for elucidating the structure-function relationships of carbohydrates and their biological significance.
Ordering Information
| Product Name | Catalog # | UNIT | Price | Qty | FAVORITES | |
Benzyl 2,3-O-Isopropylidene-L-glycero-α-D-mannoheptofuranoside, 10 mg | sc-217735 | 10 mg | $380.00 |
