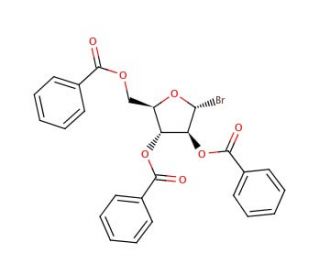
2,3,5-Tri-O-benzoyl-α-D-arabinofuranosyl bromide (CAS 4348-68-9)
QUICK LINKS
2,3,5-Tri-O-benzoyl-α-D-arabinofuranosyl bromide plays a crucial role in carbohydrate chemistry research, primarily as a versatile building block for the synthesis of arabinofuranosides and related carbohydrate derivatives. Its mechanism of action involves the activation of the anomeric carbon of the arabinose moiety through bromide leaving group, facilitating glycosylation reactions with various acceptor molecules. This chemical has been extensively utilized in the synthesis of arabinosides, glycoconjugates, and glycolipids, contributing to the development of diverse molecular probes and research tools. Researchers have employed these synthetic compounds to investigate biological processes involving arabinose-containing molecules, such as carbohydrate metabolism, host-pathogen interactions, and cell surface recognition events. Additionally, the structural diversity afforded by arabinofuranosides has led to their exploration in drug discovery efforts, particularly in antimicrobial, antiviral, and anticancer research. Moreover, studies focusing on the stereochemical and regioselective aspects of glycosylation reactions using 2,3,5-Tri-O-benzoyl-α-D-arabinofuranosyl bromide have provided insights into fundamental carbohydrate chemistry principles and synthetic methodologies, further advancing our understanding of carbohydrate structure-function relationships and paving the way for the development of novel carbohydrate-based diagnostic tools in various research domains.
2,3,5-Tri-O-benzoyl-α-D-arabinofuranosyl bromide (CAS 4348-68-9) References
- Ring opening of acylated β-d-arabinofuranose 1,2,5-orthobenzoates with nucleophiles allows access to novel selectively-protected arabinofuranose building blocks. | Podvalnyy, NM., et al. 2011. Carbohydr Res. 346: 7-15. PMID: 21109236
- Synthesis of 4′, 8-dihydroxyisoflavon-7-yl α-d-arabinofuranoside | Shiozaki, M. 1999. Tetrahedron: Asymmetry. 10(8): 1477-1482.
- Phosphorylated Glycoconjugates Based on Isosteviol, d-Arabinofuranose, and d-Ribofuranose | Sharipova, R. R., Belenok, M. G., Strobykina, I. Y., & Kataev, V. E. 2019. Russian Journal of Organic Chemistry. 55: 508-513.
- Supramer analysis of 2,3,5-tri-O-benzoyl-α-d-arabinofuranosyl bromide solutions in different solvents: supramolecular aggregation of solute molecules in 1,2-dichloroethane mediated by halogen bonds | Orlova, A. V., Ahiadorme, D. A., Laptinskaya, T. V., & Kononov, L. O. 2021. Russian Chemical Bulletin. 70(11): 2214-2219.
Ordering Information
| Product Name | Catalog # | UNIT | Price | Qty | FAVORITES | |
2,3,5-Tri-O-benzoyl-α-D-arabinofuranosyl bromide, 5 g | sc-251839 | 5 g | $471.00 |
