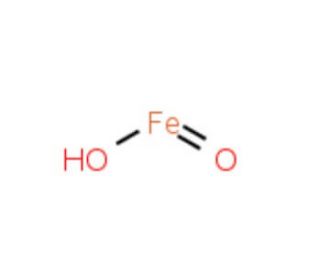
Iron(III) hydroxide (CAS 20344-49-4)
Direktverknüpfungen
Iron(III)-Hydroxid, auch als ferrisches Hydroxid bekannt, ist ein Eisenoxid-Mineral. Es kommt natürlich in Böden, Sedimenten und Gesteinen vor und ist ein wesentlicher Bestandteil von Rost. Iron(III)-Hydroxid ist weit verbreitet und spielt eine entscheidende Rolle als Eisenquelle für zahlreiche Organismen. Seine vielseitigen Anwendungen umfassen die Funktion als Katalysator in der synthetischen Kraftstoffproduktion, als Pigment in Farben und Beschichtungen und als Bestandteil bei der Herstellung von Eisen- und Stahlprodukten. Darüber hinaus wurde es zur Untersuchung der Korrosion von Eisen und Stahl eingesetzt, da es für die Rostbildung von Bedeutung ist. Geologen verwenden Iron(III)-Hydroxid, um verschiedene geologische Prozesse zu untersuchen, da es ein wesentlicher Bestandteil von Gesteinen ist. Außerdem wurde Iron(III)-Hydroxid zur Untersuchung der Auswirkungen von Umweltverschmutzung, insbesondere in Smog, und zur Bewertung der Auswirkungen des Klimawandels durch die Untersuchung seiner Anwesenheit in Staub eingesetzt. Das Wirkungsmechanismus von Iron(III)-Hydroxid ist eng mit der Chemie von Eisen verbunden. Eisen, als Übergangsmetall, zeigt komplexe Chemie mit verschiedenen Oxidationszuständen. In seiner stabilsten Form ist Eisen im +3-Oxidationszustand vorhanden, was dem Iron(III)-Hydroxid entspricht. Unter bestimmten Bedingungen kann Eisen oxidiert oder reduziert werden. Die Oxidation von Eisen führt zur Bildung von Eisenoxid-Mineralien wie Iron(III)-Hydroxid, während die Reduktion von Eisen zur Bildung von metallischem Eisen führt.
Iron(III) hydroxide (CAS 20344-49-4) Literaturhinweise
- Synergistische Wirkung der reduktiven und der durch Liganden geförderten Auflösung von Goethit. | Wang, Z., et al. 2015. Environ Sci Technol. 49: 7236-44. PMID: 25965980
- Gleichzeitige Reduktion und Sequestrierung von sechswertigem Chrom durch magnetisches β-Cyclodextrin stabilisiertes Fe3S4. | Kong, L., et al. 2022. J Hazard Mater. 431: 128592. PMID: 35247740
- Individuelles Eisen(iii)-Glycerolat: Synthese und Charakterisierung. | Khonina, TG., et al. 2022. RSC Adv. 12: 4042-4046. PMID: 35425460
- 7Be-Rückgewinnung aus Meerwasser durch verschiedene Sorbentien. | Bezhin, NA., et al. 2023. Materials (Basel). 16: PMID: 37297222
- Rückgewinnung von Radionukliden aus Meerwasser mit FIC- und FIC-A-Sorbentien. | Bezhin, NA., et al. 2023. Materials (Basel). 16: PMID: 37297315
Bestellinformation
| Produkt | Katalog # | EINHEIT | Preis | ANZAHL | Favoriten | |
Iron(III) hydroxide, 50 g | sc-363276 | 50 g | $121.00 | |||
Iron(III) hydroxide, 250 g | sc-363276A | 250 g | $413.00 |
