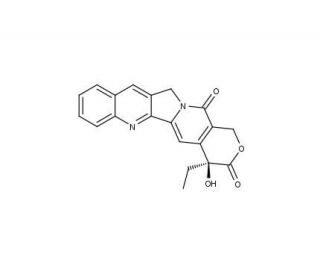
Camptothecin (CAS 7689-03-4)
Voir les citations produits (21)
ACCÈS RAPIDE AUX LIENS
La camptothécine est un composé alcaloïde trouvé à l'origine dans l'arbre chinois Camptotheca acuminata. Dans le cadre de la recherche, la camptothécine est appréciée pour sa capacité à inhiber l'ADN topoisomérase I, une enzyme essentielle à la réplication et à la transcription de l'ADN. En stabilisant la rupture transitoire de l'ADN double brin créée par la topoisomérase I, la camptothécine induit des dommages à l'ADN et finalement la mort cellulaire, ce qui est particulièrement important pour les études sur la prolifération et la viabilité des cellules. Cette caractéristique en a fait un point focal dans l'étude des mécanismes utilisés par les cellules cancéreuses pour proliférer et survivre, ainsi que dans l'exploration de stratégies potentielles pour induire une mort cellulaire contrôlée. Son rôle dans l'induction de l'apoptose par la voie de la réponse aux lésions de l'ADN contribue également à la compréhension de l'équilibre complexe entre la survie et la mort des cellules. La recherche avec la camptothécine s'étend également au domaine de la biologie moléculaire, permettant de mieux comprendre la dynamique de la réplication de l'ADN et la réponse cellulaire au stress génotoxique.
Camptothecin (CAS 7689-03-4) Références
- Distribution corporelle chez la souris de nanoparticules lipidiques solides de camptothécine injectées par voie intraveineuse et effet de ciblage sur le cerveau. | Yang, SC., et al. 1999. J Control Release. 59: 299-307. PMID: 10332062
- Distribution de la camptothécine après administration sous forme d'aérosol de liposomes ou après injection intramusculaire chez la souris. | Koshkina, NV., et al. 1999. Cancer Chemother Pharmacol. 44: 187-92. PMID: 10453719
- La camptothécine supprime la biosynthèse du monoxyde d'azote dans les macrophages RAW 264.7. | Chiou, WF., et al. 2001. Life Sci. 69: 625-35. PMID: 11476184
- Camptothécine et ses analogues: une revue de leur potentiel chimiothérapeutique. | Sriram, D., et al. 2005. Nat Prod Res. 19: 393-412. PMID: 15938148
- Piégeage irréversible du complexe covalent ADN-topoisomérase I. Marquage par affinité du site de liaison de la camptothécine. | Hertzberg, RP., et al. 1990. J Biol Chem. 265: 19287-95. PMID: 2172250
- Métabolisme et pharmacocinétique de l'analogue de la camptothécine CPT-11 chez la souris. | Kaneda, N., et al. 1990. Cancer Res. 50: 1715-20. PMID: 2306725
- Production durable de camptothécine à partir d'une Alternaria sp. isolée de Nothapodytes nimmoniana. | Mohinudeen, IAHK., et al. 2021. Sci Rep. 11: 1478. PMID: 33446714
- Études sur l'activité antitumorale, le mécanisme d'action et les effets sur le cycle cellulaire de la camptothécine. | Gallo, RC., et al. 1971. J Natl Cancer Inst. 46: 789-95. PMID: 4995657
- Stabilisation différentielle des complexes clivables de l'ADN topoisomérase I eucaryote par des dérivés de la camptothécine. | Tanizawa, A., et al. 1995. Biochemistry. 34: 7200-6. PMID: 7766631
- Statut actuel des analogues de la camptothécine en tant qu'agents antitumoraux. | Slichenmyer, WJ., et al. 1993. J Natl Cancer Inst. 85: 271-91. PMID: 8381186
- Le médicament anticancéreux camptothécine inhibe l'élongation mais stimule l'initiation de la transcription de l'ARN polymérase II. | Ljungman, M. and Hanawalt, PC. 1996. Carcinogenesis. 17: 31-5. PMID: 8565133
- L'agent chimiothérapeutique CPT-11 induit une nouvelle expression de l'initiateur de l'apoptose dans le cytoplasme. | Suzuki, A. and Kato, M. 1996. Exp Cell Res. 227: 154-9. PMID: 8806462
- Apoptose induite par la camptothécine dans les cellules de leucémie humaine HL60 sans p53 et leurs noyaux isolés: les effets des inhibiteurs de protéase Z-VAD-fmk et de la dichloroisocoumarine suggèrent une implication des caspases et des protéases à sérine. | Shimizu, T. and Pommier, Y. 1997. Leukemia. 11: 1238-44. PMID: 9264376
- Mécanisme d'action de l'ADN topoisomérase I eucaryote et médicaments ciblant l'enzyme. | Pommier, Y., et al. 1998. Biochim Biophys Acta. 1400: 83-105. PMID: 9748515
Informations pour la commande
| Nom du produit | Ref. Catalogue | COND. | Prix HT | QTÉ | Favoris | |
Camptothecin, 50 mg | sc-200871 | 50 mg | $58.00 | |||
Camptothecin, 100 mg | sc-200871B | 100 mg | $94.00 | |||
Camptothecin, 250 mg | sc-200871A | 250 mg | $186.00 |
