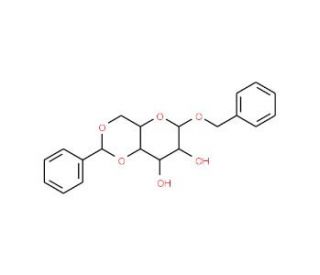
Benzyl 4,6-O-Benzylidene-β-D-glucopyranoside 의 분자 구조, CAS 번호: 58006-32-9
Benzyl 4,6-O-Benzylidene-β-D-glucopyranoside (CAS 58006-32-9)
CAS 등록번호:
58006-32-9
분자량:
358.39
분자식:
C20H22O6
연구용으로만 사용가능합니다. 진단이나 치료용으로 사용불가합니다.
* Refer to Certificate of Analysis for lot specific data.
빠른 링크
주문정보
설명
기술정보
안전정보
SDS 및 분석 증명서
벤질 4,6-O-벤질리덴-β-D-글루코피라노사이드는 당화 반응 연구를 위해 탄수화물 화학 분야에서 광범위하게 사용되는 합성적으로 변형된 포도당 유도체입니다. 이 화합물은 합성 과정에서 원치 않는 가수분해 반응으로부터 분자를 안정화시키는 보호기 역할을 하는 벤질리덴 아세탈이 4,6 위치에 있는 것이 특징입니다. 이 보호기의 존재는 당 분자의 다른 부분에 대한 선택적 화학적 변형을 촉진하는 데 매우 중요하며, 연구자들은 배당체 결합 형성을 정밀하게 제어하여 복잡한 올리고당과 글리코콘쥬게이트의 합성을 탐구할 수 있습니다. 연구에서 이 화학 물질은 주로 생물학적 시스템에서 배당체 결합의 생합성을 담당하는 효소인 글리코실전달효소의 메커니즘을 조사하는 데 사용됩니다. 이러한 효소가 벤질 4,6-O-벤질리덴-β-D-글루코피라노사이드와 같은 보호 당과 어떻게 상호작용하는지 연구함으로써 과학자들은 효소의 특이성과 반응성에 영향을 미치는 요인을 더 잘 이해할 수 있습니다. 이러한 지식은 의약품 및 생물학적 활성 화합물 생산에 필수적인 천연 글리코실화 과정을 모방한 합성 경로를 설계하는 데 필수적입니다. 이 화학물질에 대한 연구는 당과학을 발전시키고 새로운 재료와 생화학 도구를 개발하는 데 중요한 탄수화물 공학의 미묘한 부분에 대한 통찰력을 제공합니다.
Benzyl 4,6-O-Benzylidene-β-D-glucopyranoside (CAS 58006-32-9) 참고자료
- 메틸 피루베이트로 아세탈화 중 루이스 산 촉매에 의한 알킬 d-글리코피라노사이드의 이성질체화 및 재배열: 1-(카르복시에틸리덴) 글리코피라노실 공여체의 제조에 이를 활용하는 방법 | Ziegler, T., Eckhardt, E., & Herold, G. 1992. Liebigs Annalen der Chemie. 1992(5): 441-451.
주문정보
| 제품명 | 카탈로그 번호 | 단위 | 가격 | 수량 | 관심품목 | |
Benzyl 4,6-O-Benzylidene-β-D-glucopyranoside, 500 mg | sc-221338 | 500 mg | $380.00 |
