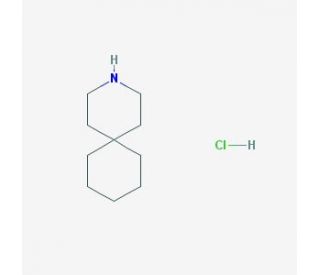
4,4-Pentamethylenepiperidine hydrochloride (CAS 180-44-9)
QUICK LINKS
4,4-Pentamethylenepiperidine hydrochloride is a chemical compound consisting of a piperidine ring — a six-membered heterocycle with five methylene groups and one nitrogen atom — substituted with a pentamethylene group at the 4-position. The structure is completed with a hydrochloride salt form, enhancing its solubility in water and other polar solvents. This compound is particularly notable in the field of organic chemistry for its role in research involving chemical synthesis and reaction mechanisms. The primary mechanism of action of 4,4-Pentamethylenepiperidine hydrochloride involves its use as a base in organic synthesis. It acts as a sterically hindered, non-nucleophilic base due to the bulkiness provided by the pentamethylene group. This steric hindrance prevents the base from participating in side reactions, making it an ideal candidate for use in various organic reactions where selectivity and control are crucial. In research, 4,4-Pentamethylenepiperidine hydrochloride is employed to study the kinetics and mechanisms of base-catalyzed reactions. It is particularly useful in reactions where a strong, non-nucleophilic base is required to deprotonate substrates without being involved in nucleophilic addition or substitution reactions. For example, it is used in the generation of carbanions from ketones, esters, and cyano compounds, where its role is to abstract a proton to form the corresponding carbanion intermediate. Moreover, this compound is used in the study of cyclic and acyclic amines to understand the effects of steric hindrance on basicity and nucleophilicity. Researchers utilize 4,4-Pentamethylenepiperidine hydrochloride to explore how the structure of amines affects their reactivity in various organic reactions, including condensation and alkylation processes.
4,4-Pentamethylenepiperidine hydrochloride (CAS 180-44-9) References
- Discovery of spiro-piperidine inhibitors and their modulation of the dynamics of the M2 proton channel from influenza A virus. | Wang, J., et al. 2009. J Am Chem Soc. 131: 8066-76. PMID: 19469531
Ordering Information
| Product Name | Catalog # | UNIT | Price | Qty | FAVORITES | |
4,4-Pentamethylenepiperidine hydrochloride, 50 mg | sc-361084 | 50 mg | $76.00 |
