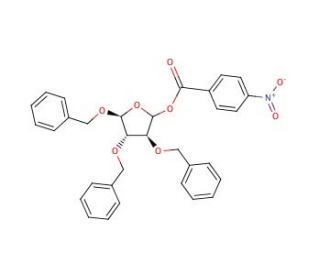
2,3,5-Tri-O-benzyl-D-arabinofuranose 1-(4-nitrobenzoate) (CAS 52522-49-3)
LINK RAPIDI
Il 2,3,5-Tri-O-benzil-D-arabinofuranosio 1-(4-nitrobenzoato) è un composto sintetico frequentemente utilizzato nella chimica dei carboidrati e negli studi di glicosilazione. La sua struttura, caratterizzata da gruppi protettivi benzilici e da un estere nitrobenzoato, è progettata per facilitare la deprotezione selettiva e le successive reazioni di glicosilazione. Nella ricerca, questo composto serve come intermedio cruciale per sintetizzare oligosaccaridi e glicosidi complessi, consentendo lo studio delle interazioni carboidrato-proteina e lo sviluppo di sonde basate sui glicani. I gruppi benzilici conferiscono stabilità durante le varie fasi di sintesi, consentendo una precisa manipolazione della frazione zuccherina. L'estere nitrobenzoato funziona come gruppo di partenza nelle reazioni di glicosilazione, aumentando l'efficienza della formazione del legame glicosidico. Gli studi hanno utilizzato questo composto per esplorare i meccanismi degli enzimi di glicosilazione, come le glicosiltransferasi, e il loro ruolo nei processi cellulari. Inoltre, il suo utilizzo per sintetizzare strutture glicaniche ha facilitato la ricerca sui glicoconiugati della superficie cellulare e sul loro coinvolgimento nella segnalazione, nell'adesione e nel riconoscimento delle cellule. Consentendo la costruzione di strutture glicaniche definite, il 2,3,5-Tri-O-benzil-D-arabinofuranosio 1-(4-nitrobenzoato) contribuisce in modo significativo a far progredire la nostra comprensione della glicobiologia e dei ruoli funzionali dei carboidrati nei sistemi biologici.
2,3,5-Tri-O-benzyl-D-arabinofuranose 1-(4-nitrobenzoate) (CAS 52522-49-3) Referenze
- Fotossigenazione sensibilizzata da coloranti di furani furanosilici: sintesi di un nuovo nucleoside C piridazina. | Cermola, F., et al. 2005. J Org Chem. 70: 6503-5. PMID: 16050717
Informazioni ordini
| Nome del prodotto | Codice del prodotto | UNITÀ | Prezzo | Quantità | Preferiti | |
2,3,5-Tri-O-benzyl-D-arabinofuranose 1-(4-nitrobenzoate), 5 g | sc-256302 | 5 g | $128.00 |
