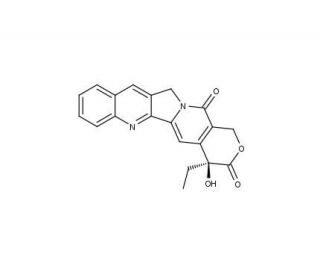
Camptothecin の分子構造、CAS番号: 7689-03-4
Camptothecin (CAS 7689-03-4)
参考文献をチェックします (21)
別名:
(S)-(+)-Camptothecin
アプリケーション:
Camptothecinは抗腫瘍活性を示す可逆的ミトコンドリアトポイソメラーゼI阻害剤です
CAS 番号:
7689-03-4
純度:
≥98%
分子量:
348.4
分子式:
C20H16N2O4
補足情報:
これは輸送上の危険物に分類され、追加の送料が発生する場合があります。
試験・研究用以外には使用しないでください。 臨床及び体外診断には使用できません。
* Refer to Certificate of Analysis for lot specific data.
クイックリンク
注文情報
参考文献
説明
技術サポート情報
安全情報
SDSと分析証明書
カンプトテシンは、もともと中国の樹木Camptotheca acuminataから発見されたアルカロイド化合物である。研究の文脈では、カンプトテシンはDNAの複製と転写に重要な酵素であるDNAトポイソメラーゼIを阻害する能力で評価されている。トポイソメラーゼIが作り出す二本鎖DNAの一過性の切断を安定化させることにより、カンプトテシンはDNA損傷を誘発し、最終的には細胞死を引き起こす。この特性により、カンプトテシンは、がん細胞が増殖と生存のために用いるメカニズムの研究や、制御された細胞死を誘導するための潜在的な戦略の探求における焦点となっている。DNA損傷応答経路を通じてアポトーシスを誘導するカンプトテシンの役割は、細胞の生存と死との間の複雑なバランスの理解にさらに貢献している。カンプトテシンの研究は分子生物学の分野にも及んでおり、DNA複製の動態や遺伝毒性ストレスに対する細胞応答についての洞察を提供している。
Camptothecin (CAS 7689-03-4) 参考文献
- カンプトテシン固体脂質ナノ粒子の静脈内注射によるマウスの体内分布と脳へのターゲティング効果。 | Yang, SC., et al. 1999. J Control Release. 59: 299-307. PMID: 10332062
- リポソーム・エアゾールとして投与した後, またはマウスに筋肉内注射した後のcamptothecinの分布。 | Koshkina, NV., et al. 1999. Cancer Chemother Pharmacol. 44: 187-92. PMID: 10453719
- カンプトテシンはRAW 264.7マクロファージにおける一酸化窒素生合成を抑制する。 | Chiou, WF., et al. 2001. Life Sci. 69: 625-35. PMID: 11476184
- カンプトテシンとその類似体:化学療法の可能性に関する総説。 | Sriram, D., et al. 2005. Nat Prod Res. 19: 393-412. PMID: 15938148
- DNA-トポイソメラーゼI共有結合複合体の不可逆的トラップ。カンプトテシン結合部位の親和性標識。 | Hertzberg, RP., et al. 1990. J Biol Chem. 265: 19287-95. PMID: 2172250
- マウスにおけるカンプトテシンアナログCPT-11の代謝および薬物動態。 | Kaneda, N., et al. 1990. Cancer Res. 50: 1715-20. PMID: 2306725
- Nothapodytes nimmonianaから分離されたAlternaria sp.からのcamptothecinの持続可能な生産。 | Mohinudeen, IAHK., et al. 2021. Sci Rep. 11: 1478. PMID: 33446714
- カンプトテシンの抗腫瘍活性, 作用機序および細胞周期効果に関する研究。 | Gallo, RC., et al. 1971. J Natl Cancer Inst. 46: 789-95. PMID: 4995657
- 真核生物DNAトポイソメラーゼI切断性複合体のカンプトテシン誘導体による安定化の違い。 | Tanizawa, A., et al. 1995. Biochemistry. 34: 7200-6. PMID: 7766631
- 抗腫瘍剤としてのカンプトテシン類似体の現状。 | Slichenmyer, WJ., et al. 1993. J Natl Cancer Inst. 85: 271-91. PMID: 8381186
- 抗癌剤カンプトテシンは, RNAポリメラーゼII転写の伸長を阻害するが, 開始は刺激する。 | Ljungman, M. and Hanawalt, PC. 1996. Carcinogenesis. 17: 31-5. PMID: 8565133
- 化学療法剤CPT-11は, アポトーシス開始因子の細胞質への新たな発現を誘導する。 | Suzuki, A. and Kato, M. 1996. Exp Cell Res. 227: 154-9. PMID: 8806462
- p53欠損ヒト白血病HL60細胞とその分離核におけるカンプトテシン誘導アポトーシス:プロテアーゼ阻害剤Z-VAD-fmkとジクロロイソクマリンの効果は, カスパーゼとセリンプロテアーゼの両方の関与を示唆している。 | Shimizu, T. and Pommier, Y. 1997. Leukemia. 11: 1238-44. PMID: 9264376
- 真核生物DNAトポイソメラーゼIの作用機序と酵素を標的とする薬剤。 | Pommier, Y., et al. 1998. Biochim Biophys Acta. 1400: 83-105. PMID: 9748515
の阻害剤である:
(eukaryotic translation initiation factor) eIF, (eukaryotic translation initiation factor) eIF1, (eukaryotic translation initiation factor) eIF2, (eukaryotic translation initiation factor) eIF3, (eukaryotic translation initiation factor) eIF4, (eukaryotic translation initiation factor) eIF5, α-Adaptin 2, α-Dystrobrevin, α3e Tubulin, α8 Tubulin, β-SNAP, 2410076I21Rik, 2610002J02Rik, 4632434I11Rik, 4921530L21Rik, 4930432K21Rik, 4930469G21Rik, 4930547C10Rik, 4931408C20Rik, 6030422M02Rik, 9130206N08Rik, A630018P17Rik, A630023A22Rik, ACD, ACTBL2, ACTR5, ADAM1, ADAT2, Adducin α, ADSL, AGAP3, AI481877, AK4, Alix, ALMS1, ALS, ANKRD12, ANKRD13D, ANKRD18A, ANKRD32, ANKRD54, Annexin A13, Annexin V, Aprataxin, ARD1B, arfaptin 1, ARGLU1, ARL8A, ARMC1, ARMC3, ASAH2C, ASB-17, ATAD5, ATP5L, ATP5L2, ATP9BL, ATPBD3, ATXN3L, ATXN7L3, B930041F14Rik, BAFL, Barx1, BC005561, BC049762, BFZB, BJ-TSA-9, Blood Group N antigen, BMAL2, BMCP1, BOLA1, BRCC3, BRE, Brn-3c, BRP44, BTBD11, BTEB3, BTF3L2, BTF3L4, BTF3L4P, BUD13, C10orf28, C10orf50, C10orf51, C10orf75, C11orf82, C11orf91, C13orf30, C14orf124, C14orf72, C15orf42, C16orf62, C17orf39_4933439F18Rik, C17orf53, C18orf18, C19orf57, C1D, C1orf114, C1orf127, C1orf55, C20orf112, C20orf43, C2orf49_AI597479, C3orf24, C4BPα, C5orf21, C6orf134, C6orf167, C6orf48, C8orf13, C8orf45, C9orf142, CACNG6, CALML6, calsyntenin-3, CARF, CARP-1, CAS4, CCDC4, CCDC57, CCDC66, CCDC79, CCDC98, CCR-9, Cdc45, CDRT15L, CELF5, CENP-B, CENP-L, CENP-S, CHD (chromodomain helicase), CHD7, CHMP1A, Chondrolectin, CIZ1, claudin-20, CMPK, CMTM2a, CMTM2b, Cnbp2, COH1, COL12A, COL9A2, colostrinin, CPEB, CRALBPL, Creatininase, CRISP-10, CrxOS, cryptdin 6, CSDA, CSHL1, Csprs, CST9L, CstF-64T, CstF-77, CTAGE5, CTGLF3, CUL-5, Cux2, CWF19L1, CWF19L2, CXorf39, CXorf57_D330045A20Rik, cyclin O, D930020E02Rik, DBR1, DCAR, Dclre1c, DDX16, DDX24, DDX30, DDX31, DDX35, DDX38, DDX3L, DDX42, DDX52, DDX54, DEAH-box RNA-dependent NTPase (DHX), Dematin, DFF-45, Dim1, DinB, Diversin, DNA Ligase I, DNA Ligase III, DNA pol δ 2, DNA pol δ cat, DNA pol ε B, DNA pol ε cat, DNA pol ε ss, DNA pol λ, DNA pol μ, DNA pol ν, DNA pol ζ, DNA pol 4, DNA2L, DNAHC7B, DND1, DYDC1, Dynlt1e, Dynlt1f, E130309D02Rik, E430018J23Rik, Ear12, Ear7, EG210583, EG210853, EG232801, EG245174, EG245263, EG384589, EG435970, EG546272, EG546282, EG625591, EG628359, eIF1AY, eIF2Bδ, eIF2C2, eIF3M, eIF4B, eIF5A1, ELAC1, Elongin B, ENSMUSG00000073257, ERI-1, ES31, ESCO2, ETAA16, EXDL1, EXTL2, F730047E07Rik, Factor XIII B, FAM108A1, FAM131A, FAM139A, FAM186A, FAM64A, FAST, FER1L6, Fhit, FIGNL2, Filensin, FIV p27, FLJ20433, FLJ43860, FLRT3, FOXI1, FTF, gametogenetin, GAR1, GCNF, Geminin, GFRP, GGNBP1, Ggnbp2, Gm22, GNL2, GOLGA8B, GPSN2, GRINL1A, GS27, GTSE-1, Gucy2d, Gvin1, GW182, H1FOO, H2-M10.4, H2-M2, H2-Q2, H2-T24, H2AL2, H2BFA, Hairy, HAP1A, hCAP-D2, hCG_2015138, hCG_2024596, HcRed, hDcp1a, hDcp2, HEATR5A, HEI10, HEL308, HELIC1, HELIC2, Hfm1, Histatin 1, Histone cluster 1 H2AF, Histone cluster 2 H2BF, Histone cluster 2 H3C, Histone cluster 2 H3D, Histone cluster 2 H4A, Histone cluster 2 H4B, Histone cluster 3 H2A, Histone cluster 3 H2ba, HLA-3, HLTF, HMG-1L1, HMG-I/HMG-Y, HMG20A, HMGI-C, hMLH3, hMSH3, hnRNP A0, hnRNP B1, hnRNP H, hnRNP H′/hnRNP H2, hnRNP M, HNRNPA1L2, Hop2, HPA1, HPR, HPV 16-L1, hSNF2H, HSPC144, HSV-2 gB, hunchback, I-SceI, I830077J02Rik, IFITM3, IFITM6, IFT43, IGHMBP2, Importin-8, Ini1, INO80B, INO80E, INOC1, INTS2, INTS5, IQCF4, ITPase, Jaz, JK-1, Junctophilin-1, Keratin 42, Khdc1c, KIAA0460, KIAA1462, KIAA1530, KIN17, KLH-A, Klk1b3, Klra33, KRT222P, KRTAP10-8, KRTAP11-1, KRTAP14, KRTAP16-7, KRTAP9-L3, LBA1, Lce1a2, Lce1l, LCTL, LDH-AL6A, LEMD1, LEO1, LFY, LIN-54, Lipocalin-11, Lipocalin-5, Liprin α1, LOC100039248, LOC100039284, LOC100039560, LOC100039633, LOC100039786, LOC100039830, LOC100039934, LOC100040201, LOC100040214, LOC100040307, LOC100040682, LOC100040839, LOC100040885, LOC100040981, LOC100041019, LOC100041106, LOC100041119, LOC100041179, LOC143666, LOC198437, LOC255130, LOC284395, LOC285423, LOC345630, LOC388436, LOC388820, LOC441258, LOC441461, LOC642361, LOC643770, LOC643862, LOC644277, LOC645967, LOC646762, LOC727967_BOP1, LOC729978, LOC729991, LRRC55, MA1, MAGP-2, MBP-probe, MCCB, MCFD2L, MCM-BP, MCM3AP, MDGA2, Mec3, MEIOB, MFSD11, MGC87042, Mi2-β, Miner2, Mitochondrial Topo I, mKIAA1530, MLZE, MMR2, MMS19, MND1, MOBKL1A, MOCS1, Morc, Mpa2, MPZL1, MREG, MRT-2, MTP18, MUP17, MUP18, MUS81, MYLC2PL, Myosin VI, N10, NC2α, Ncapd3, Ncapg, NELF, NETO2, NF90, NHP2, Nibrin, Nocturnin, NOL55, NOP16, Nop5, Nop58, NOS, NOS2, NOSTRIN, NPAT, NSE4A, NTH1, Oatp-Y, OBFC1, OBFC2A, OBFC2B, OBP-2A, Odf1, OFCC1, Olfr176, Olfr177, Olfr417, Olfr523, Olfr987, Olfr988, Olr619, OR10H2, OR4C45, OR51AA4, OR52I2, OTTMUSG00000010657, OTTMUSG00000010671, OTTMUSG00000011290, OTTMUSG00000016414, OTTMUSG00000016437, OTTMUSG00000016779, OTTMUSG00000016780, p54/nrb, p91, PABPC1L, PACT, PAI-3, pancpin, Pancreasin, PAPSS 1, PC-PLD4, PDE6C, pescadillo, PGBD1, PheRS, PIF1, PIG-B, PIG-Y, PLC-XD1, PLGLB1, PLK4, PMS1, PMS2, POLDIP2, POLR3E, POM121, Pontin 52, POP5, POTE14, POTEJ, PRDM11, PRIM2A, Protamine 1, PRP19, PRR17, Psf1, Psf3, PSG9, PUS1, quiescin Q6, Rab 22A, Rad53, Rad55, Rad59, RAET1G, Ran, RASAL3, Raver1, Raver2, RBM10, RBM16, RBM19, RBM27, RBM32B, RBMX2, RBMXL3, RBMY1F, RBP1, RCC2, RCCD1, RCOR3, RecA, RECA-1, REF2, REPIN1, REPS1, REXO1, RFC, RFC2, RFC3, RFPL3, Rhox2d, Ribosomal Protein L12, Ribosomal Protein L34, Ribosomal Protein S29, Ribosomal Protein S8, Rim4, RIMBP3C, RMI1, RNF170, RPA 34 kDa subunit, RPA16, RPA40, RPAC2, RPL7A, Rpp14, Rpp20, Rpp38, RPS4X, RQCD1, Rrn3, RRP36, RRS1, Rsf-1, RSL1D1, RUFY4, RuvA, RuvC, RWDD2A, RYBP, SA-2, SAS10, scc-112_Pds5a, Schlafen (SLFN) family, SCML4, SCP-3, Sec24D, SEI-1, Separase, Septin 8, SerpinB6, SETX, SFRS2B, Sgs1, SKIV2L2, Slfn2, Slfn3, SLIRP, SLX1A/B_SLX1, Sm, SMC4, SMC5, SMC6, Smt3, SNAT4, SNM1A, SNM1B, Snrp116, SP140L, SPAG11B, Spartan, SPATA12, Spc24, Spc25, SPF30, SPIN90, SPLUNC3, SPNR, Spo11, SPT16, SPTLC3, ss DNA marker, SSB-4, SSNA1, ST8Sia II, STAG3, Statherin, SUN2, Sval3, SWI/SNF-B, Swi1, SWI5, SYCE1, Sycp2l, Syntaxin 18, TAF I p110_TAF I p95, TAF I p48, TAF II p100, TAF5L, TAFII-28, TASK-2, TBL3, TCTE1, TDE1, TDP1, TDP2, TDRD9, Testican-1, TEX11, TEX15, TFEC, TFIIA, TFIIA-β, TFIIA-γ, TFIID, TFIIE-α, TFIIF RAP 74, TFIIH 2C, TFIIH p8, TFIIIB, TFIIIB90, TFIIIC35, TFIIS, THAP2, THAP9, THOC3, THOC4, THTR2, TIAR, Timm8a2, TISP74, TMCO5B, TMEM16D, TMEM33, TMEM40, TMEM45A, TMEM91, TMPRSS7, TMUB1, TNFα-IP 8L1, Tom40, Top3, Topo, Topo I, Topo IIIβ, TOPORS, TP53TG5, TRABD, TRAG-3, TRAP150, TREX-2, Trim12c, TRIM30C, TRIM35, TRIP, Tropomyosin α, Tropomyosin β, TRPD52L3, TSC-22 D4, TSHZ3, TSLP, TSPAN1, TSR1, TTC21A, TTC22, TTI2_BC019943, TUSC2, Twinfilin-1, Txl-2, TYW4, UBF, UBF1, Ubr4, UDG2, UIMC1, UNC5H4, UNC84A, UPIb, V1RH17, VCX, VCX-B, VCX-C, VCY, VDAC1, VEST1, Vgl-4, Vitrin, VPS13C, VWA5A, WDFY3, WDHD1, WDR18, WDR21A, WDR57, Wnt-8b, WWC2, XPF, XRCC1, XRCC2, XRCC4, XRCC6BP1, YFP, ZBTB24, ZBTB8B, ZC3H12B, ZCCHC4, ZCCHC7, ZFF29, ZFP108, ZFP109, ZFP11, ZFP110, ZFP113, ZFP119, ZFP141, ZFP192, ZFP213, ZFP26, ZFP37, Zfp870, ZNF100, ZNF33A, ZNF33B, ZNF510, ZNF596, ZNF746, ZNF772, ZNF818, ZRANB3, ZSCAN4.のアクティベーター:
4930547C10Rik, 53BP1, A1-d, AIF, Aladin, ALKBH2, Apaf-1, APLF, APRIN, Arp9, ASF1A, ASF1B, ATR, Baxα, BC055324, BCLAF1, BinCARD, Bir1, BLM hydrolase, Bmf, BNIP-2, BNIPL-2, BRAF35, BTBD12, Bub2, C15orf41, C1orf227, C20orf196, C20orf43, C230052I12Rik, C330006K01Rik, CAD, caspase-3, caspase-8, CCDC6, CCDC76, Cdc13, Cdc25B, Cdc25C, Cdc2B, Cdc50C, CDCA2, Cdt2, CED-3, CED-6, CED-7, CED-8, CENP-S, Chk1, Chk2, Cid, Clb5, CPP32 p11, CPSF3, CPSF4, CRM1, Crt1, CTF8, CtIP, CTR9, cyclin δ-3, cyclin 2b, cyclin 3a, Cyclon, CYFIP2, D19Bwg1357e, DAP-3, DCC1, Dclre1c, DDB2, DDI1, DDI2, DDX1, DDX26B, DDX46, DEDD, DEDD2, DEM1, Diablo, Diva, DNA pol α, DNA pol ε A, DNase II, DR4, Dtl, EAF2, EG194588, EG240038, EG244495, EG333452, EG408191, EG434881, EGL-1, EI24, Elm1, Eme1, ENDOG, EndoV, ERCC1, Esp1, EST-1, EWS, EXOD1, FAM128A, FANCA, FANCD2, FANK1, FASP1, FHAD1, FIV gp36, FLASH, FLJ20433, Fnk, GADD 45β, GADD 45γ, GEN1, gp210, granzyme H, GTPBP8, H2bl2, Haspin, hCAP-G, Hfm1, Histone cluster 1 H2AE, Histone H2A.J, Histone H2A.X, hnRNP L, hnRNP X, HORMAD1, Hus1B, INO80C, Kar9, karyopherin α6, KCTD19, KIAA0406, Kin28, KRR1, Ku, La/SSB, LIN-35, LOC100041244, LOC641776, LOC643862, LOC643909, LOC729991, LRRC3B, LRWD1, MAGOHB, Mat1, MBD4, MCM5, MCM9, MCPyV large T-antigen, MDC1, MEI1, MLH3, MRE11_Mre11, MSH3_Msh3, MUS81, MYBBP1A, NAP1, Nek1, Nek8, NIRF, NSE1, NSE2, OR2G2, ORC2_Orc2, ORC3, OTTMUSG00000010552, OTTMUSG00000010657, OTUB1, p19 ARF, p53, p53CSV, p53R2, p73β, PA28α, PABP, PABPC2, PABPC6, PAR-4, PARP-1, PARP-8, Pdcd2L, PGBD3, PGBD5, PITSLRE A, PNK, POL H, Pol III RPC39, Pol2, POLA2, POLR2G, POLR2I, PQBP-1, Psf2, PSR, Rad23, Rad24, Rad26L, Rad3, Rad5, Rad51, Rad51B, Rad51D, Rad52, Rad57, Rad9, Rad9B, RAP80, Ras GAP, RBM4B, RecQL1, RecQL5, REV1, Rev3, REXO2, RFC, RFC1, RFC4, RFC5, RFWD3, RMI1, RMI2, RNase HII-A, RNase HII-C, RNase III Drosha, RNF113B, RNF168, RNF169, RP11-430L17.1, RP11-592B15.4, RPA 70 kDa subunit, RPRD1B, RREB1, RRP22, SFRS12, SFRS2B, SKIV2L, SLX1A/B_SLX1, Smac, SmarcAL1, SMC1α, SMC1β, SNM1B, SPACRCAN, SPATA22, SPATA5L1, SPHAR, SR140, SRp75, Srs2, SSBP4, String, Tβ-15b, TATDN2, TATDN3, TDRD3, TESSP2, TF, TFIIE-β, TFIIH p34, TGIF2LX1, Top1, Topo IIIβ, TP53INP1, TP53TG3, TP53TG5, TRIM46, Trp53i11, Trp53i13, TTC36, TTI1, TTP, U11/U12 snRNP 20K, UBE2T, UDG, USP51, VPRBP, Wee 1, Wip1, XPC, ZBTB37, ZNF347, ZNF440, ZNF8, ZW10.注文情報
| 製品名 | カタログ # | 単位 | 価格 | 数量 | お気に入り | |
Camptothecin, 50 mg | sc-200871 | 50 mg | $58.00 | |||
Camptothecin, 100 mg | sc-200871B | 100 mg | $94.00 | |||
Camptothecin, 250 mg | sc-200871A | 250 mg | $186.00 |
